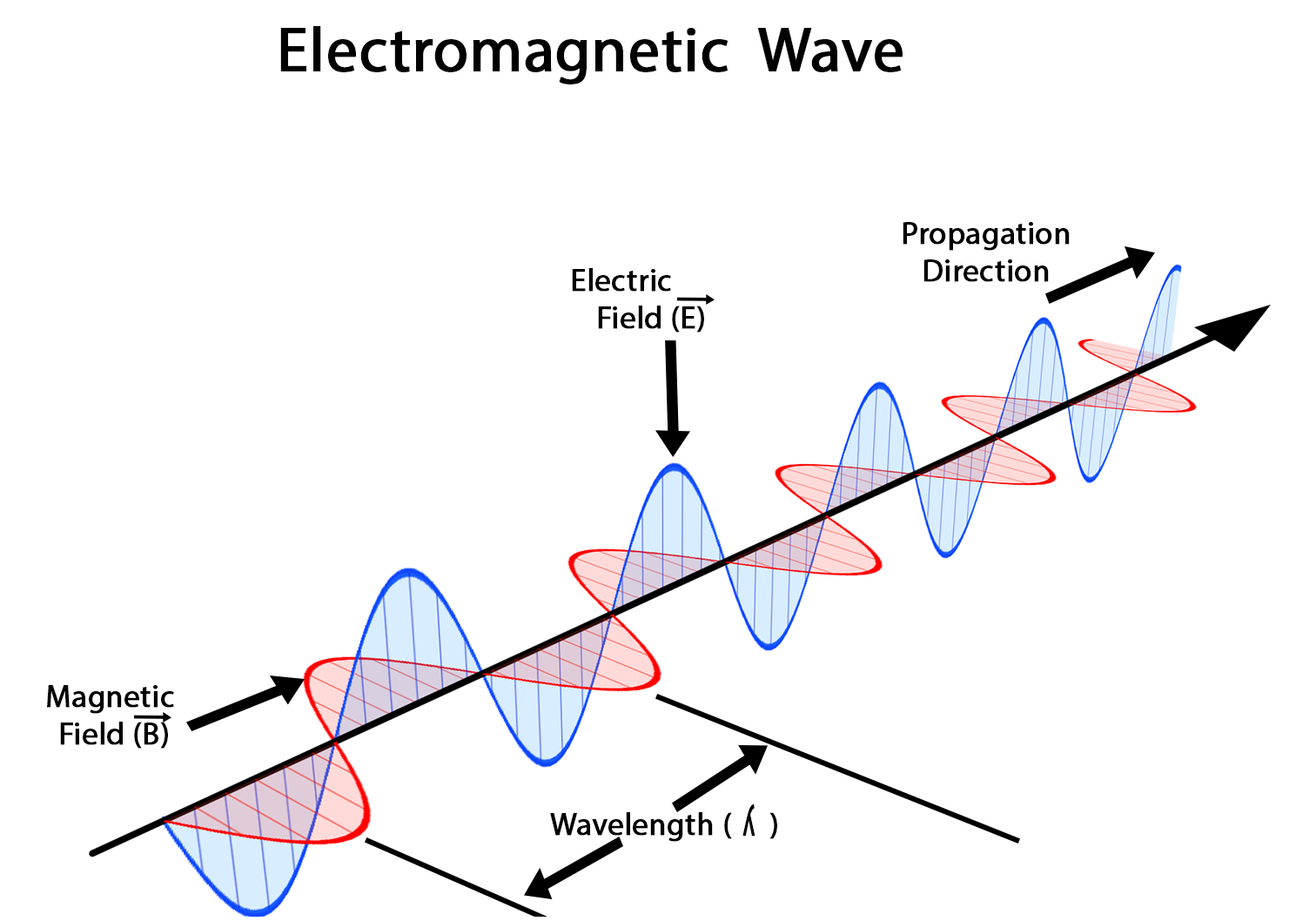
Electromagnetic waves have another name: "light". Light in nature is just some energy carried by an electrical wave and a magnetic wave together, as illustrated below. The electrical wave and the magnetic wave are perpendicular to each other, and they travel in the same direction which defines the direction of the electromagnetic wave.
Illustration of electromagnetic wave (credit: wikipedia)
Electromagnetic waves have a few characters. Let's discuss them one by one.
First, speed (\( c \)). All electromagnetic waves travel at the same speed, and yes, that's just the speed of light. In vacuum, light travels at a speed of \( 2.998 \times 10^8 \pu{m/s} \).
Second, wavelength (\( \lambda \)). Wavelength is defined as the distance between the two adjacent peaks of the wave. In chemistry, we use the unit meter (\( \mathrm{m} \)) for wavelength.
Different electromagnetic waves have different wavelengthes. For example, the microwave used in the microwave oven has a different wavelength as compared to visible lights. Even for the visible lights themselves, they have different wavelengthes as red light has a longer wavelength as compared to violet light.
Thus we can arrange different electromagnetic waves in the sequence of their wavelengthes, and that's called electromagnetic spectrum (as shown below).
Electromagnetic spectrum (credit: wikipedia)
You may notice that visible lights only exist in a very narrow region in the whole electromagnetic spectrum. Yes, that's true. Human eyes are only capable of seeing a very small range of lights, while some animals are able to see lights in a much wider wavelength regions. Thus, the words "visible lights" only tell us that humans are able to see these electromagnetic waves. Visible lights are nothing special but just some electromagnetic waves that happen to be seeable by our naked eyes. They are exactly the same as all other electromagnetic waves that we can't see. So, don't be fooled by the word "visible" or "light".
Now we want to introduce the third character of electromagnetic waves, namely frequency (\( \nu \) or \( f \)). Frequency simply means how many peaks we would see in one second, when the electromagnetic wave is travelling past us.
Since all electromagnetic waves travel at the same speed (i.e. the speed of light), you could imagine, if the distance between two peaks are longer (longer wavelength), the time taken for the second peak to reach us will also be longer, and hence fewer peaks we could see in one second (lower frequency)
In mathematics, we would describe the relationship between wavelength (\(\lambda\)) and frequency (\(\nu\)) as follows:
\[ c=\lambda\times\nu\ or\ \nu=\ce{\frac{c}{\lambda}} \]
As we could see from the equation, wavelength and frequency are inversely proportional. Thus, longer the wavelength, lower the frequency and vice versa.
In chemistry, the unit of frequency is \( s^{-1} \) or \( \mathrm{Hz} \). \( 1\mathrm{Hz} \) just means \(1s^{-1} \), or 1 peak you'll see in one second.
Energy of light
Before we discuss the fourth character of electromagnetic waves, we need to understand a new concept, called photon which could be understood as a packet of electromagnetic waves.
We don't have to understand what a photon is, or how it behaves under different circumstances. We could simply say that for example, when a lamp is emitting light and our eyes are seeing the light, the light is not like a rope hanging between the lamp and our eyes. In fact, there're many small "balls" flying from the lamp to our eyes, and each ball is carrying some electromagnetic waves inside it. And we call these balls as photons. Just remember, this is an oversimplified metaphor and does not represent the true physics behind photons. But as chemistry students, we're not required to know too much about that, just leave it to physicists. =)
So just put it really simple. Lights do not travel in a continuous pattern. Instead, they get into small groups (or photons), and move one group at a time.
Now let's move on to learn about the energy of lights. We all know we could get sunburn if we stand in the sun for too long. That's because sunshine carries energy and hurts our skins, or to be more specific, it's the UV light in sunshine that hurts our skins. But why UV light?
Remember when we learnt about frequency of light? It turns out that the energy of lights (\(E\)) is proportional to their frequencies.
\[ E\propto\nu \]
Of course we're not satisfied with this proportional relationship only. We want to calculate the exact numbers. In order to do that, we use Planck's constant.
\[ E=h\nu=h\times\ce{\frac{c}{\lambda}} \]
where \( h=\pu{6.626E-34}\ \mathrm{m^2\cdot kg\cdot s^{-1}}\), and \( E \) has a unit of \(\mathrm{J}\).
This equation allows us to calculate the energy of a single photon. Remember, lights are made of photons. We can't really calculate energy of lights, because they can be bright or dim, but we are able to calculate energy of a single photon.
Be more careful with the units. Sometimes we use \(\mathrm{kJ}\) for some properties, and sometimes we use \(\mathrm{J}\) like in this case. So if you're doing any calculations, remember to check the units after each step.
Electromagnetic spectrum
Let's talk a bit more about the electromagnetic spectrum. Although all electromagnetic waves are just lights in nature, we still put them into different categories so that we could study them one by one.
On the leftmost side of the spectrum, we have Gamma rays, which have very short wavelengths and high frequencies. As we have learnt that energy of light is proportional to its frequency, we would know that Gamma rays are having ultra high energy. That's why they are able to penetrate into our body and cause harms to our cells.
X rays are having lower energy than Gamma rays, but still very high. As a result, we could use X rays to generate images about our bones and tissues. However, X rays are still harmful to our body, especially if we are exposed to X rays in large doses or for a long period of time. Hense usage of X rays is greatly monitored and regulated.
Next, we have ultraviolet (UV) lights on the right hand side of X rays in the spectrum. UVs have higher energy as compared to the visible lights, and are responsible for sunburns.
UV sanitization
During the Covid-19 pandemic period, people are using different ways of sanitization. UV lamps are being widely used especially in venues such as shopping malls, airports, and offices. These lamps generate UV lights which could damage the protein shells of the virus. However, it is essential to ensure that the UV lights could shine on the target surfaces, because UV lights are not able to penetrate or maneuver in different directions.
However, it's not recommended to use UV lamps at home. UV lights may cause damages to our skins and eyes if not handled properly. So it is better to be operated by trained personnels.
The narrow region between UV and infra-red (IR) lights has the visible lights. When we see the white lights, they are actually composed of lights with different wavelengths. Roughly we could categorize visible lights into violet, blue, green, yellow, orange, and red colors. But do take note that there is no clear distinguish or separation lines between different colors. All the lights with different colors are in fact blending into each other, and the color change is very gradual. We'll come back to this in more details later.
Going towards the right hand side of the spectrum, we then have IR, microwaves, and radio waves. They have relatively low energy, and thus are safe to use in our daily life. For example, we use microwaves in the microwave ovens, and we use radio waves to carry broadcasts in the air. The currently trending 5G signals for our mobile phones are utilizing microwaves as well.
Questions
Alright, now we have learnt about electromagnetic waves and electromagnetic spectrum. We know the properties of electromagnetic waves, such as speed (\(c\)), wavelength (\(\lambda\)), frequence (\(\nu\ or \ f\)), and energy of photons (\(E\)) too, and we're able to calculate these properties. So now, we'll leave you some homework, hope you could get all of them correct.
-
Which one has higher energy? Visible light with a wavelength of 500nm or microwave with a frequency of 4.7GHz?
-
How much energy in J does one photon of the 4.7GHz microwave carry? (\( 1\mathrm{GHz} = 10^9 \mathrm{Hz} \))
-
When a photon with a wavelength of 91nm transfers all its energy to an electron in a hydrogen atom, the electron could escape from the hydrogen atom. 1) Calculate the energy that is being transfered in this scenario, and the wavelength of the photon. 2) What kind of electromagnetic wave is the photon?