Why do we learn about subatomic particles anyway? We said that atom is the smallest unit during a chemical reaction, remember? Then why do we bother to know what's inside an atom?
Alright, that's because subatomic particles would affect how an atom behaves during a chemical reaction. By studying subatomic particles, we would understand, or even predict, how atoms would break up, recombine and give rise to new substances. And that is the top priority for chemists.
First, let's talk about protons.
Elements
Scientists have found more than 100 types of atoms so far. By saying "types of atoms", we are actually saying atoms with different number of protons. Atoms with the same number of protons are defined as an element, which is going to behave the same way during a chemical process. In this regard, the number of protons tells us the identity of an atom, and we call that as atomic number.
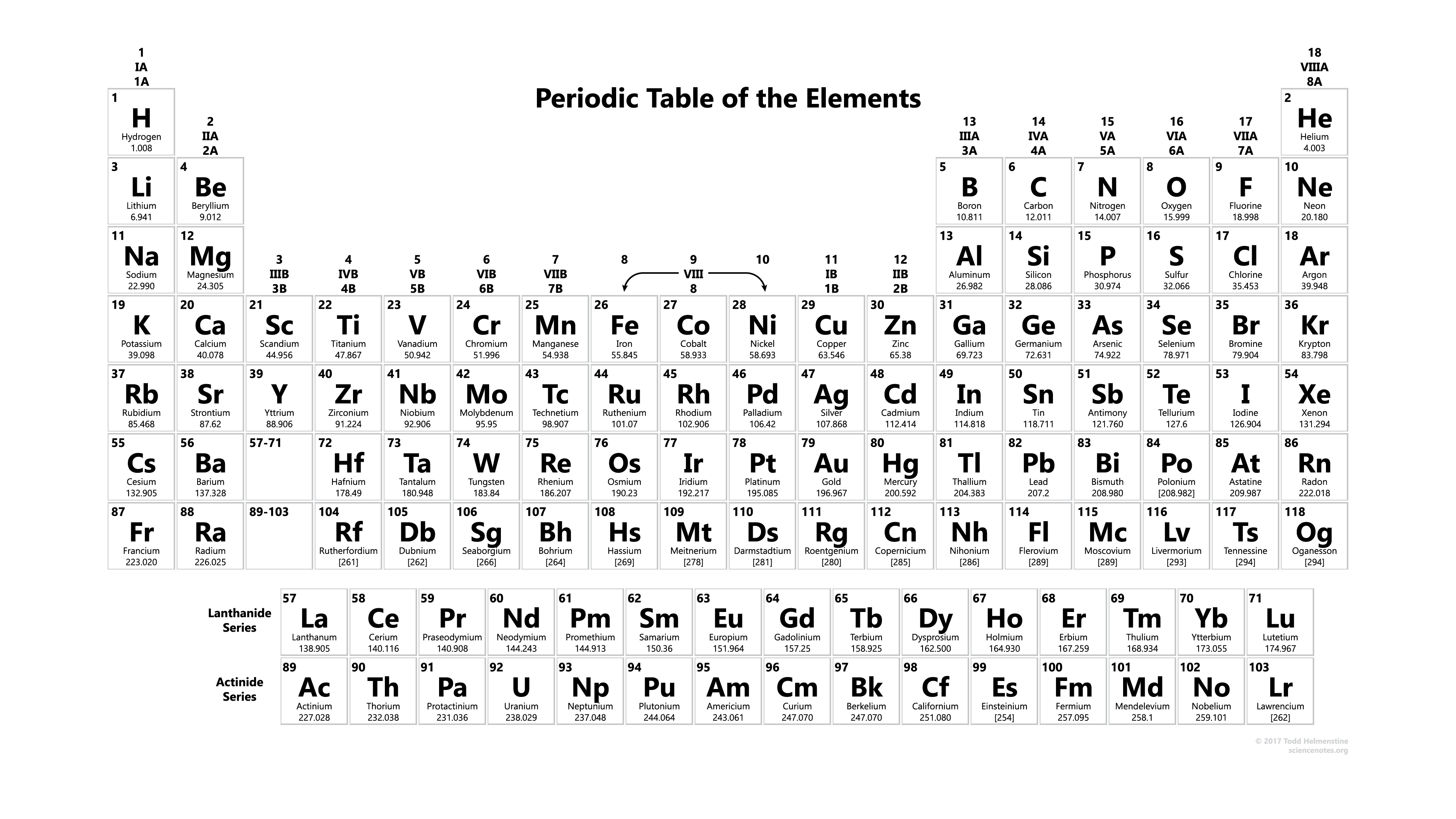
Periodic table
Periodic table is a summary of all the elements found to date. Elements are arranged in the sequence of increasing atomic number in the periodic table. You may find the symbols of the elements (consider the symbols as abbreviates to their names) and other informations. We'll talk about the periodic table in more details later. For now, you may just have a general idea what a periodic table looks like.
Elemental substance
In chemistry, the word element could also refer to any substance that is made of atoms of the same element (or elemental substances). Confused? Well, let's put it this way: element means atoms with the same number of protons, as well as the substance that these atoms will naturally form without the presence of any other type of atoms. For example, letter O in chemistry stands for an element named oxygen, and when two oxygen atoms naturally combine together to form oxygen gas (\(\ce{O2}\)), we say the gas \(\ce{O2}\) is an element too.
Elemental substances or elements could not be separated into two or more different substances, neither with physical methods nor chemical processes.
Compounds
As opposed to elements, a compound is consisting of atoms of different elements. For example, water, which is written as \(\ce{H2O}\) in chemistry, is made of 2 hydrogen atoms (\(\ce{H}\)) and 1 oxygen atom (\(\ce{O}\)).
Compounds could be separated into two or more different substances using chemical processes, but not with physical methods.
Please don't mistaken the concept of element and compound for that of molecule. A molecule is a particle made of more than one atom, while a compound is a substance whose molecules contain more than one type of atoms (or its molecules are made of atoms of different elements). As you may have noticed, an element could also exist as molecules just like the oxygen gas does. Oxygen gas molecule \(\ce{O2}\) is made of two oxygen atoms so it is definitely a molecule, but since there is only one type of atom (\(\ce{O}\)), it is not a compound.
Mixtures
Despite of having multiple types of atoms in the molecules of compounds, compounds are still considered pure substance, which is defined as a substance formed by a single type of molecule. There are however a lot of things that are made of different type of molecules, which we call them as mixtures. For example, the moment you inhale, you taking a mixture "air", which contains 20% oxygen gas (\( \ce{O2} \)), and 80% nitrogen gas (\( \ce{N2} \)). The oxygen molecules and nitrogen molecules do NOT bind together to form a single molecule. Oxygen gas molecules are produced by plants and are moving to the area where there's no plant, but nitrogen gas molecules do not have such movements. Therefore, we can say that nitrogen and oxygen have totally seperate and different molecules, and air is a mixture of oxygen gas and nitrogen gas.
Another example would be a cup of salt water. When you add some table salt to a cup of water, you are making a mixture. If you taste this cup of salt water, you'll not notice any difference from the top of the water as compared to the bottom of the water. For a mixture like this, we say it is a homogeneous mixture, meaning it will have uniform composition and properties.
But when you mix a bowl of salad, you may see more leaves on the top, and tomatos may be at the bottom. For this kind of mixtures, we call them heterogeneous mixture, where you'll find nonuniform composition and properties through out the volume of the mixture.
In short, elements and compounds are pure substances, and they can form homogeneous mixtures or heterogeneous mixtures when different molecules of elements or compounds mix without forming a new type of molecule.
Mixtures could be separated into its constituents with physical changes only.
We shall elaborate more on compounds and mixtures soon.