In previous posts we have already seen this symbol many times, \[ \ce{_1^1H} \] which we used to represent one of the isotope, hydrogen-1. Let's understand the meaning of this nuclear symbol.
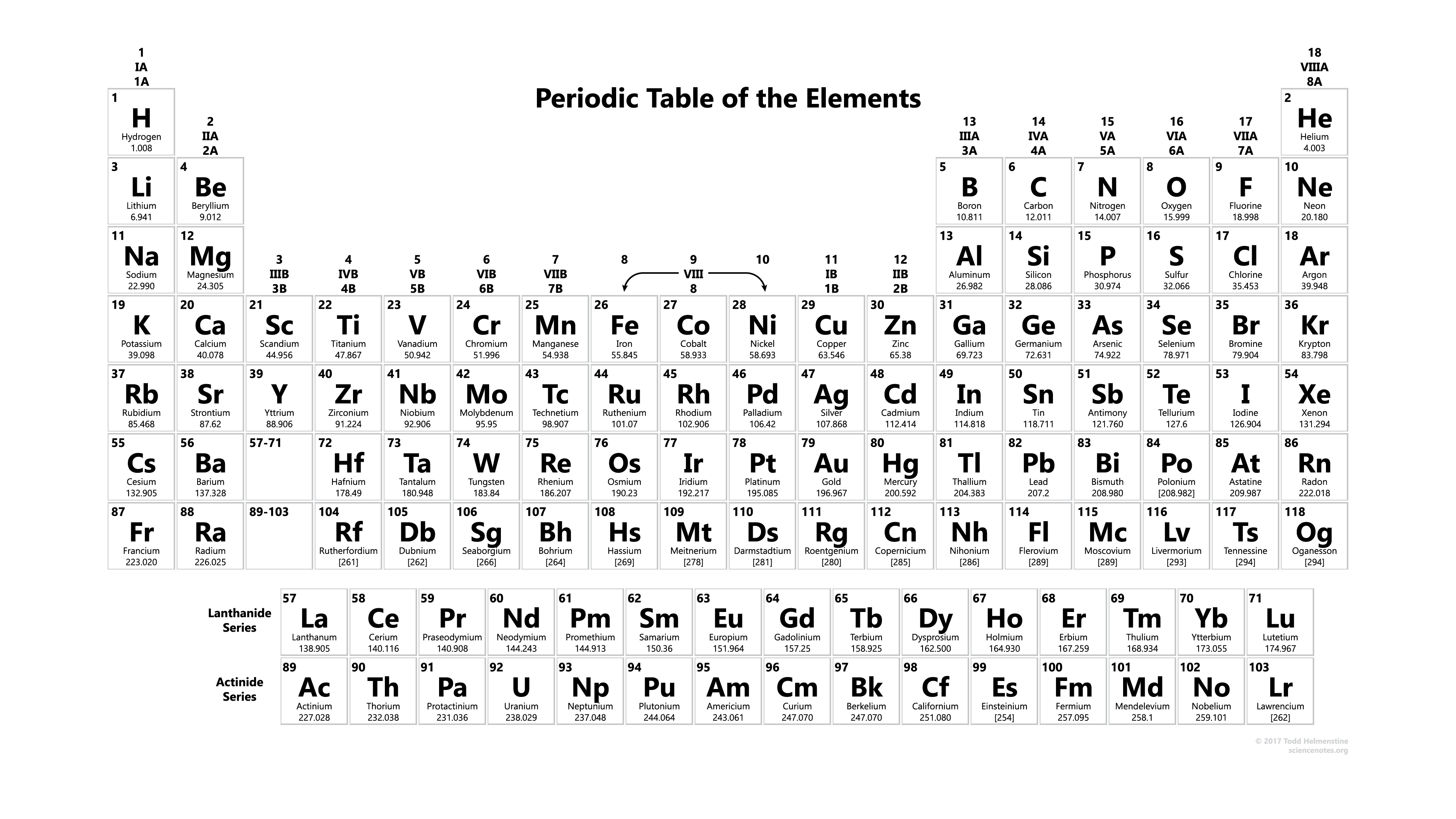
Periodic table
First, let's focus on the H. This is the symbol representing hydrogen. All elements have their own symbol which is shown in the periodic table.
There are a lot of information on the periodic table, and we'll look at the symbols of elements for now.
In the periodic table, you'll find, for example, hydrogen in the top-left corner, represented by H. You could also find O, which is in the second row and the third box from the right, representing oxygen. For you reference, you may also find the names of the elements below the symbols. But please be more familiar with the elements in the first 4 rows, including their symbols and names.
Atomic number
In the top-left corner of the boxes in the periodic table, you may find these little numbers from number 1 until number 118. This is called the atomic number, which is identical to the number of protons in the atoms of the particular element. In previous post, we have said that the number of protons in the atom defines the identity of that atom, so the atomic number is just like the passport number of the atom, and it tells us what element the atom belongs to.
Number of protons in an atom = its atomic number
In the nuclear symbol, you'll find the atomic number of the atom in the bottom-left corner. Please be very careful with the locations. The atomic number in the periodic table is shown in the top-left corner of the box, while it is in the bottom-left corner of the nuclear symbol.
You may have noticed that periodic table simply shows all elements in the sequence of increasing atomic number.
There's one more piece of information that we can get from the atomic number. That is, the number of electrons in a neutral atom. Neutral atom means there's no net charge on the atom. But we say they have protons in the nucleus, which bring positive charge to the atom. Are we wrong? The explanation is that atoms also have electrons which have the same among of charge as protons, but their charge is negative. Therefore, to maintain a charge neutrality of the atoms, we simply need the same number of electrons as the protons.
In a neutral atom, number of electrons = number of protons
Please take note that differenter versions of periodic table has different ways of presenting the informations. For example, some periodic tables would show atomic number at the bottom. Usually these periodic tables would also include a separate box as a key or example to let you know how to interprete the table. So don't be surprised to see a different periodic table. But of course the sequence of elements and all other relevant information will not change in modern periodic tables. Only the way of presentation could vary.
Mass number
The other number in the nuclear symbol, which is in the top-left corner, is called mass number, which is the sum of number of protons and number of neutrons in the atom.
For example, \( \ce{_1^1H} \) tells us that there're 1 proton, while the total number of proton and neutron is also 1. In other words, we do not have any neutron in this particular atom (isotope). It is absolutely fine to have zero neutron in an atom. In fact, most of the hydrogen atoms on the Earth have no neutron. However, all atoms will definitely have at least one proton because, again, number of proton defines what element the atom belongs to.
Just another example, \( \ce{_8^16O} \) is yet another nuclear symbol. First of all, the letter O tells us this is an oxygen atom. The number 8 in the bottom-left corner tells us it has 8 protons in its nucleus, which makes it an oxygen. The number 16 in the top-left corner shows that it has in total 16 protons and neutrons. So we do a simple math here, \( 16 - 8 = 8 \). So we get 8 neutrons in the nucleus. As simple as that.
Number of neutrons in an atom = its mass number - its atomic number
Using the nuclear symbol, we can easily tell people what isotope we have. Of course, most of the time we do not want to differentiate different isotopes because they always exist in a particular proportion. We study atoms by considering all isotopes in the lump. In that case, we may not show the mass number.
Meanwhile, we may not show the atomic number either. This is because the symbol is already telling us what element it is, and all atoms of the same element are having a unique number of protons which we can simply read from the periodic table. There's no need for us to show the atomic number together with the symbol of the element. That's just a bit redundant. So usually you'll see things like \( \ce{H2O} \), \( \ce{O2} \), \( \ce{CH3CH2CH3} \), etc. The numbers here tell us how many of the atoms before the number are present in the molecule, rather than telling us how many protons in the atom after the number. For example, in \( \ce{H2O} \), we have 2 \( \ce{H} \) atoms and 1 \( \ce{O} \) atom in 1 \( \ce{H2O} \) molecule. The number 2 clearly doesn't mean we have 2 protons in \( \ce{O} \) which should have 8 protons. We'll talk more about these things which we call as formulae in the near future.
For now, you just need to know that, usually we use the symbol for the element alone without any number to represent an atom. Only when we want to clearly show a particular isotope, we'll have to show the mass number with or without the atomic number.
Question
Now, let's do a simple exercise. Can you fill up the blanks in the following table?
| Number of Protons | Number of Neutrons | Number of Electrons | Nuclear symbol |
|---|---|---|---|
| 5 | 6 | ||
| 16 | 18 | ||
| 7 | 7 | ||
| \( \ce{^35Cl} \) | |||
| 12 | \( \ce{^?Mg} \) |